Clinical Data Sharing Policy
Surgical Revolutions and Artificial Intelligence adopts the ICMJE clinical data sharing policy.
- As of 1 July 2018 manuscripts submitted to ICMJE journals that report the results of clinical trials must contain a data sharing statement as described below.
- Clinical trials that begin enrolling participants on or after 1 January 2019 must include a data sharing plan in the trial's registration. The ICMJE's policy regarding trial registration is explained above. If the data sharing plan changes after registration this should be reflected in the statement submitted and published with the manuscript, and updated in the registry record.
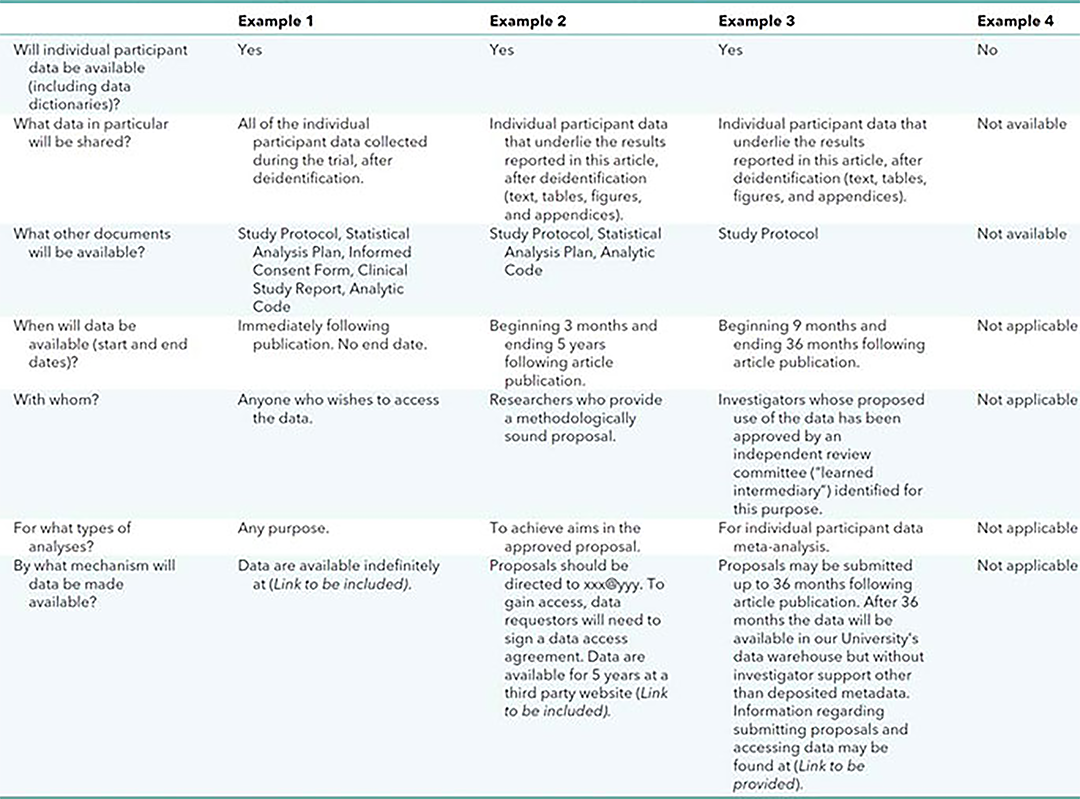
Data sharing statements must indicate the following: whether individual deidentified participant data (including data dictionaries) will be shared (“undecided” is not an acceptable answer); what data in particular will be shared; whether additional, related documents will be available (e.g., study protocol, statistical analysis plan, etc.); when the data will become available and for how long; by what access criteria data will be shared (including with whom, for what types of analyses, and by what mechanism). Illustrative examples of data sharing statements that would meet these requirements are provided in the Table.
Table 1. Examples of data sharing statements that fulfill these ICMJE requirements*
|
ICMJE = International Committee of Medical Journal Editors. *These examples are meant to illustrate a range of, but not all, data sharing options. |
Authors of secondary analyses using shared data must attest that their use was in accordance with the terms (if any) agreed to upon their receipt. They must also reference the source of the data using its unique, persistent identifier to provide appropriate credit to those who generated it and allow searching for the studies it has supported. Authors of secondary analyses must explain completely how theirs differ from previous analyses. In addition, those who generate and then share clinical trial data sets deserve substantial credit for their efforts. Those using data collected by others should seek collaboration with those who collected the data. As collaboration will not always be possible, practical, or desired, the efforts of those who generated the data must be recognized.


